The US Healthcare market is the largest in the world and within this the cancer diagnostics market is estimated at USD 56.5bn in 2022 and expected to grow at a compound annual growth rate (CAGR) of 8.4% until 2030 (Source; Precedence Research).
Early detection and good treatment are considered critical in the fight against cancer. Against this backdrop, Oxford BioDynamics PLC, has the potential to be a huge success over the coming years. This week they announced a £5.6m placing and up to a further £2m raise through a PrimaryBid offer open until 4.30pm on Thursday 3 August.
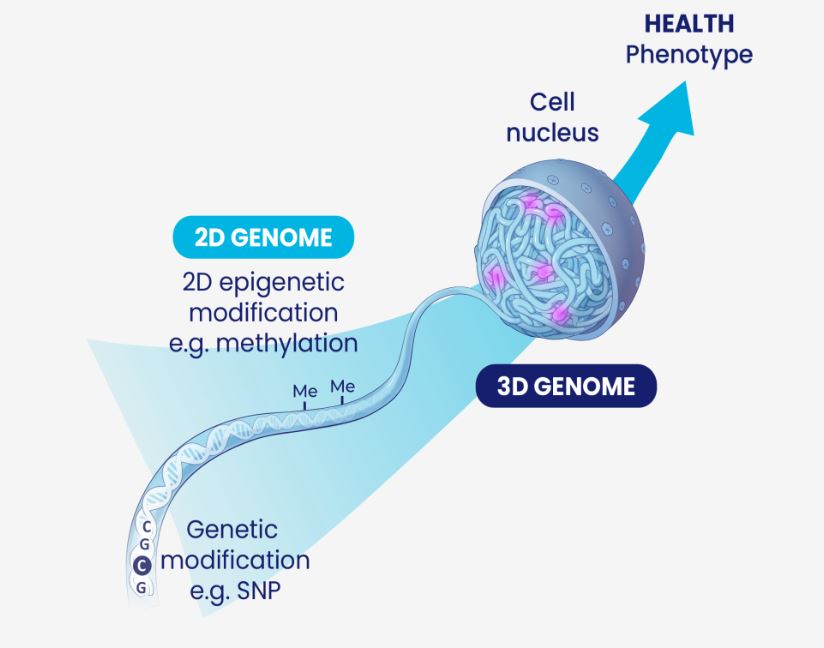
Taking a step back, Oxford BioDynamics was founded in 2007 as a spin-out from the University of Oxford by a team including its Chief Scientific Officer Dr Alexandre (“Sasha”) Akoulitchev. Oxford BioDynamics is a global leader in the field of 3D genomics, interrogating the 3D structure of the genome to reveal critical health information. The genome’s 3D architecture is as important as the genetic code it contains: it is key to the regulation (turning on and off) of genes.
Building on over 16 years of research, Oxford BioDynamics is now successfully commercializing their world-leading understanding of 3D genomics, using their proprietary automated fast turnaround blood testing technology platform, EpiSwitch®, to develop and launch validated, commercial, smart clinical tests. The technology is fully developed and protected by 18 patent families. Tests developed using EpiSwitch can be used for prediction of response to therapy, patient prognosis, disease diagnosis and disease monitoring. There is a more detailed explanation with some great videos on the Oxford Biodynamics website here.
The company strategy is centred on the development and commercialisation of precision medicine tests for cancer and other life-changing diseases.
The company launched their flagship product, the EpiSwitch CiRT (Checkpoint Inhibitor Response Test) for cancer in February 2022 and their next commercial product will be the Prostate Screening Episwitch (PSE) test for prostate cancer, which is planned to be launched in Q4 2023. The most likely next tests to follow these are a diagnostic test for the early detection of colorectal cancer and, in veterinary medicine, a diagnostic/prognostic test for various canine cancers. All of these have large potential end markets.
The EpiSwitch CiRT, which predicts a patient’s likely response to immune checkpoint inhibitor (ICI) therapies, offers significant potential benefits to patients, doctors and healthcare…